To read the full paper,
Cytostatic Hypothermia and Its Impact on Glioblastoma
A Physical Approach to a Biological Problem
Most current cancer therapies target specific molecular pathways, but tumors often adapt and become resistant. In contrast, this study explores temperature as a fundamental physical variable that broadly influences biology.
Hypothermia has long been used in medicine, but cancer-related applications have focused almost exclusively on cryogenic freezing, which indiscriminately damages both tumor and healthy tissue. In contrast, noncryogenic hypothermia takes advantage of the brain’s resilience to moderate cooling. In fact, temperatures in the low 30s Celsius can even be neuroprotective.
Based on this, “cytostatic hypothermia” can be defined as a range of temperatures that safely halts cell division without causing widespread tissue damage.
Defining a Cytostatic Temperature Window
Effects on Cell Cycle, Metabolism, and Signaling
To understand how cytostatic hypothermia affects tumor biology, its impact on cell cycle progression, metabolism, and inflammatory signaling was examined.
They observed that hypothermia caused cells to accumulate in the G2 phase of the cell cycle, indicating arrest before division. Metabolic activity was broadly reduced, including decreases in glucose consumption, lactate production, and glutamate metabolism.
In addition, production of inflammatory cytokines such as IL-6 and IL-8, which are associated with glioblastoma invasiveness, was significantly reduced under hypothermic conditions.
Importantly, these changes occurred across multiple pathways simultaneously, consistent with the authors’ argument that temperature acts as a global regulator of cellular activity.
Compatibility With Other Therapies
The interaction between cytostatic hypothermia and existing therapies was also evaluated.
When combined with the standard chemotherapy drug temozolomide, hypothermia further reduced tumor growth, including in a glioblastoma cell line known to be resistant to chemotherapy.
They also examined CAR T-cell immunotherapy in vitro. While immune activity was reduced under hypothermia, CAR T cells were still able to kill tumor cells, particularly when cooling was applied intermittently.
These findings suggest that cytostatic hypothermia may be compatible with other therapeutic approaches, though the authors emphasize that further optimization is required before combining treatments in vivo.
Delivering Hypothermia Inside the Brain
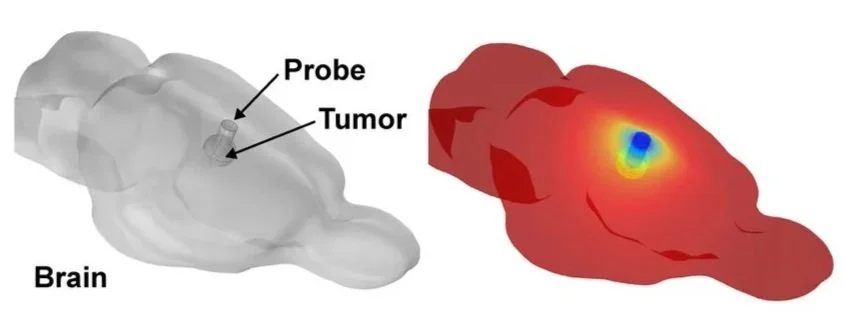
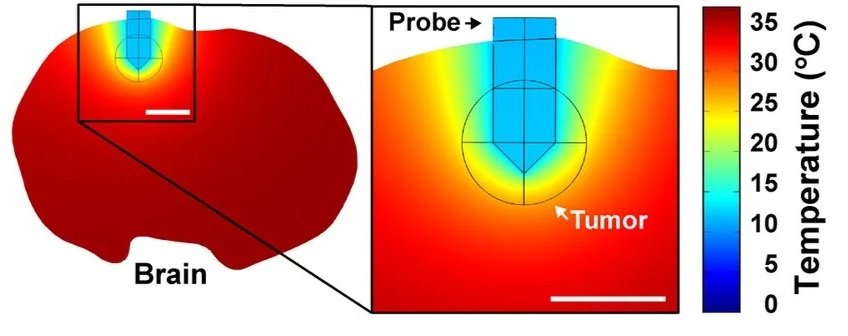
To translate these findings beyond the laboratory, an experimental system was designed to deliver localized intracranial hypothermia.
Using computational modeling based on bio-heat equations and brain perfusion parameters, they demonstrated that a small cooling probe could create a localized temperature gradient sufficient to bring tumor tissue into the cytostatic range while leaving surrounding brain tissue near normal temperature.
They then built a thermoelectric cooling system consisting of an implantable interface and a removable external cooling unit, allowing animals to remain awake and freely moving during treatment.
In Vivo Studies: Tumor Growth and Survival
Why Cytostatic Hypothermia Is Different
Unlike targeted molecular therapies, cytostatic hypothermia leverages fundamental physics that influences biology broadly. By simultaneously modulating multiple cellular pathways, it may reduce opportunities for tumor adaptation and resistance.
This physical, non–species-specific mechanism may improve the likelihood of translating results from animal models to humans.
Looking Forward
This work provides proof of concept that cytostatic hypothermia can halt glioblastoma growth and extend survival in vivo. The authors propose that future efforts focus on developing a fully implantable, patient-centric cooling device and evaluating this approach as a treatment option for patients with recurrent or treatment-resistant glioblastoma.
Cytostatic hypothermia represents a previously unexplored therapeutic approach that expands the limited set of options currently available for glioblastoma.
Glioblastoma (GBM) remains one of the most aggressive and treatment-resistant brain tumors. Despite surgery, radiation, and chemotherapy, median survival remains limited, and most tumors recur locally near their original site. Because of this high rate of local recurrence, there is a strong rationale for therapies that act directly at the tumor location rather than systemically.
This work investigates using non-freezing hypothermia to halt tumor growth, rather than attempting to destroy tumor tissue outright
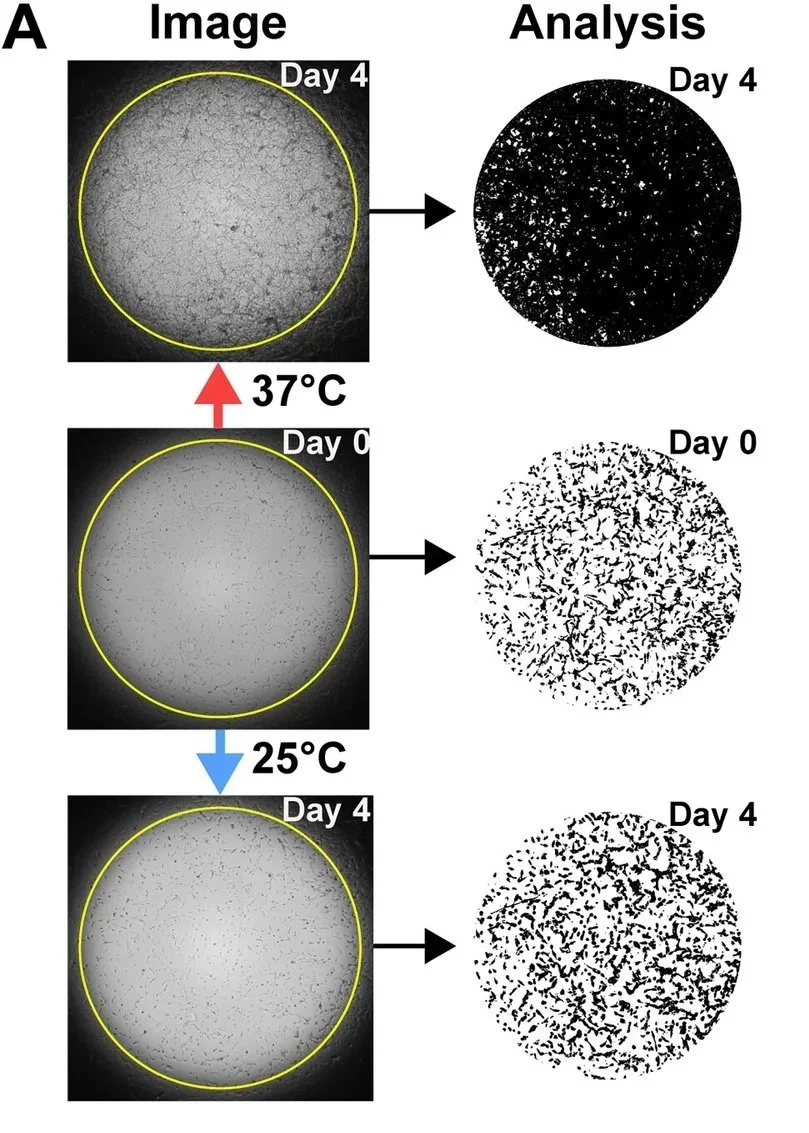
The study begins with extensive laboratory experiments using multiple human glioblastoma cell lines and a rat glioma line. Cells were grown at different temperatures to determine how cooling affects tumor growth.
Across all tested glioblastoma cell lines, the following pattern was observed:
All cell lines grew rapidly at normal body temperature (37°C)
Growth slowed at 30°C
At 25°C, human glioblastoma lines showed no growth
The rat glioma line required slightly deeper cooling, with growth halted at 20°C
20–25°C is a cytostatic window, where tumor cell division is arrested rather than cells being immediately killed.
Notably, intermittent cooling was also effective. For some cell lines, cooling for as little as 18 hours per day was sufficient to halt division, suggesting practical flexibility in how treatment might be delivered.
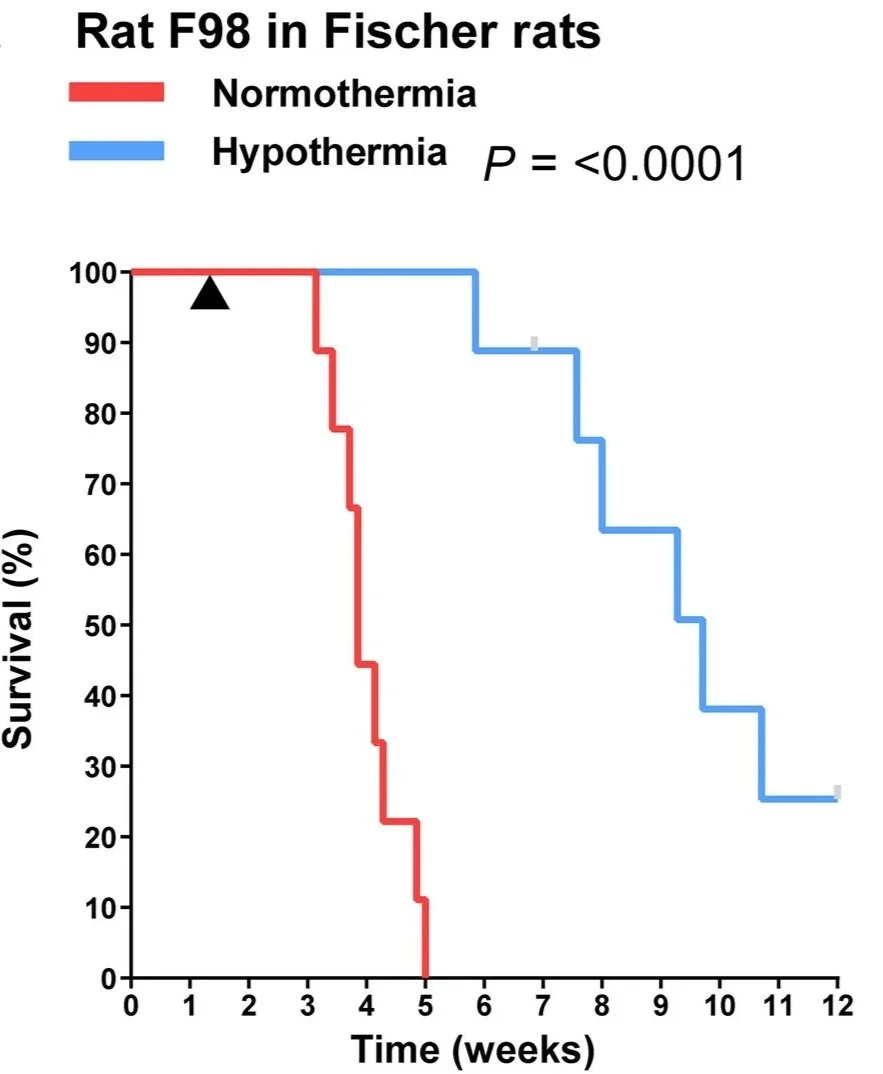
The device was tested in two rodent models of glioblastoma: an aggressive rat glioma model and a human glioblastoma xenograft model.
Across both models, cytostatic hypothermia:
Reduced tumor growth
More than doubled median survival
Allowed animals to eat, move, and behave normally during treatment
In animals where cytostatic temperatures were successfully achieved, tumors were either absent or reduced to small, nonproliferative remnants. Histological analysis showed intact neurons and glial cells in surrounding brain tissue, with no evidence of widespread damage.
All animals in which cytostatic temperatures were successfully achieved survived the duration of the study.